The Way You Move: Tumor Cells Move Differently Than Normal Ones
 By Frank Otto
By Frank Otto

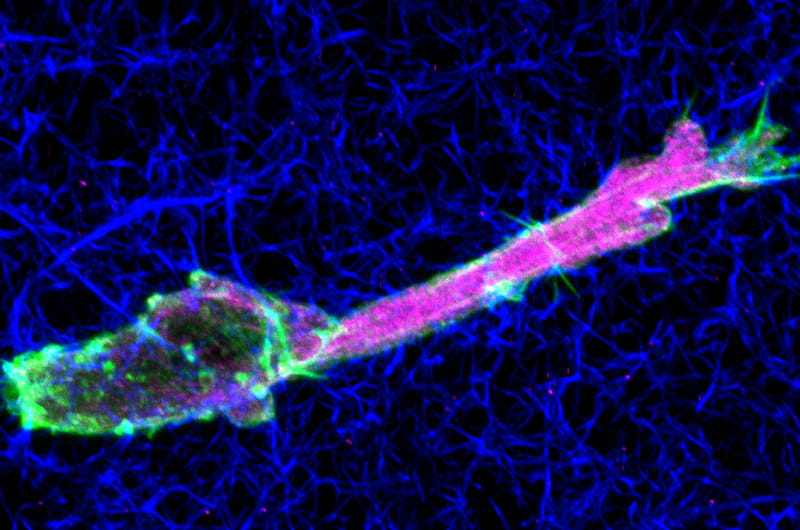
What makes cancer so deadly is its ability to move. The better that doctors can keep tumors contained and protect unaffected organs in the body, the less lethal a cancer will be.
So if doctors were able to pinpoint tumor cells and literally stop them in their tracks, it would go a long way in treatment.
“Therapeutically preventing the inappropriate movement of metastatic tumor cells could be used in combination with existing chemotherapies to increase patient survival,” said Ryan Petrie, PhD, an assistant professor in Drexel’s College of Arts and Sciences.
With that in mind, Petrie led research efforts that were able to determine that certain tumor cells (fibrosarcoma) are unable to perform a certain kind of movement that normal connective tissue cells perform when moving through tight, three-dimensional environments.
Since cells’ nuclei are big and rigid, they’re not easy to squeeze through three-dimensional structures. When such a structure (or matrix, as they’re called at the cellular level) is encountered, normal cells can switch to a form of movement that creates a pressure differential inside the cell by moving their nucleus, like a piston in an engine.
But Petrie’s research found that fibrosarcoma cells can’t perform this piston movement to get through those tight squeezes when certain protease enzymes are present and highly active. Thus, these tumor cells effectively chew their way through the matrix, while normal cells use powerful molecular motors to muscle their way forward and leave the matrix more intact.
Petrie’s research, published in the Jan. 2 issue of the Journal of Cell Biology, studied this movement of cells — and lack thereof — in rat tail and cattle skin collagen.
“Cell migration is a lethal characteristic of metastatic tumors, where malignant cells begin to move inappropriately and spread through the body to form secondary tumors,” Petrie said. “To fully understand the mechanisms which drive normal and pathological cell movement, we must study cell migration in three-dimensional environments, such as the ones found in our tissues.”
The research has implications beyond just fighting cancer cells.
“Promoting movement of fibroblasts in specific three-dimensional tissues like dermis [skin] and cartilage could help to heal difficult-to-treat wounds,” Petrie explained. “Understanding the fundamental molecular mechanisms driving the movement of these cell types will be essential for designing rational therapeutic strategies in the future.”
Determining the different methods in movement between normal and malignant cells is important, but more research needs to be done to find out exactly why there is a difference.
“The next step will be to untangle the intracellular signaling pathways which dictate cell behavior to understand precisely why tumor and normal cells move differently in the same three-dimensional environment,” Petrie said. “We speculate these signaling pathways will provide the best candidates for drugs aimed at promoting or reducing cell movement in the future.”
Those interested in reading Petrie’s full study, “Activating the Nuclear Piston Mechanism of 3D Migration in Tumor Cells,” can access it here.
In This Article
Contact
Drexel News is produced by
University Marketing and Communications.